TransCon PTH
Designed to replace parathyroid hormone to physiologic levels in adults with hypoparathyroidism
Designed to replace parathyroid hormone to physiologic levels in adults with hypoparathyroidism
Parathyroid hormone (PTH) plays a critical role in controlling calcium, phosphate, and calcitriol (active vitamin D) levels in the blood and bone. Through its primary actions, PTH directly involves the kidneys, bone, and other organs in maintaining key biological functions.
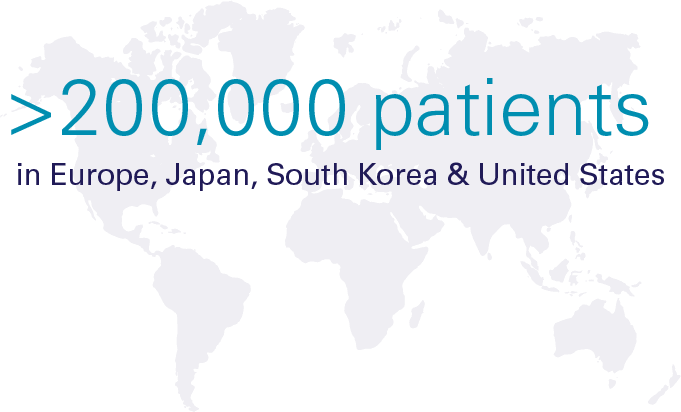
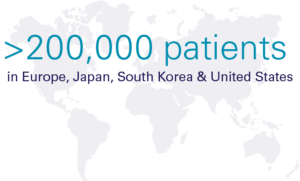
Hypoparathyroidism (sometimes called "hypopara" or "HP") is a rare endocrine disorder characterized by deficient or absent parathyroid hormone. It affects an estimated 200,000 people worldwide, most of whom develop the condition following damage to or accidental removal of the parathyroid glands during thyroid surgery. Current standard of care treatment with oral calcium and calicitriol does not effectively address the short-term symptoms, long-term complications, or quality-of-life impacts of hypoparathyroidism.
Significant Global Prevalence
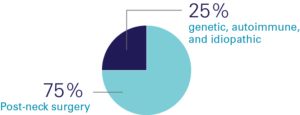
Causes of Hypopara
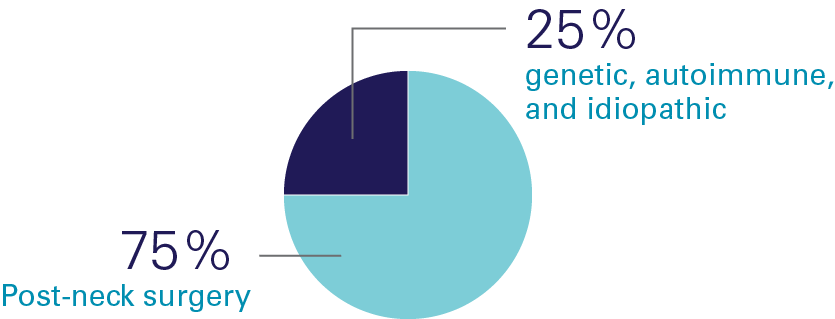
Burden of disease
People living with hypoparathyroidism experience a diverse range of physical, mental, cognitive, and emotional symptoms. They often suffer debilitating symptoms and the condition can severely impact quality of life and overall productivity, including ability to work and perform household activities.
The current standard of care with supplementation of calcium and calcitriol or the analogue alfacalcidol/alphacalcidol does not fully control the condition, poses a significant daily pill burden, and contributes to risk of long-term complications, including renal disease. People with hypoparathyroidism have at least a four-fold greater risk of kidney failure than the general population, in part due to excessive urinary calcium excretion and long-term complications caused by standard treatment.
Hypoparathyroidism is also linked to increased hospitalizations and emergency room and urgent care visits. In some cases, calcium levels fall so low that patients experience a ‘calcium crash’ and urgently require calcium delivered intravenously – a situation often dreaded by patients, their friends and family.
A survey of people living with hypoparathyroidism (N=146) showed patients required multiple physician visits and up to one decade to diagnose their condition.
A complex patient experience
![]()
Hypocalcemia (low calcium in the blood)
- Abnormal tingling, burning, numbness (paresthesia)
- Brain fog (impaired judgment, memory loss, headache)
- Muscle cramps (tetany)
- Seizures
- Laryngospasm (spasm of the voice box)
Hypercalcemia (too much calcium in the blood)
- Muscle weakness, coma, constipation, urination difficulties
Anxiety/fear
- May relate to potential calcium crash
![]()
Calcification
- Calcium deposits in the kidney, brain, blood vessels, eye, and other soft tissues
Kidney disease
- 4- to 8-fold increased risk of kidney disease compared to healthy populations
Depression or bipolar disorder
- 2-fold increased risk of depression or bipolar disorder compared to healthy populations
![]()
Negative psychological impacts
- 100% of patients said their condition interferes with their daily lives
Impacts on physical functioning
- 85% reported an inability to perform household activities
Negative impacts on the ability to work or work productivity
- 76% reported their condition interfered with work productivity
- 30% were no longer able to work
TransCon PTH
TransCon™ PTH is a prodrug of parathyroid hormone (PTH) designed to restore physiologic levels of PTH 24 hours a day. By doing so, we believe TransCon PTH can address both the short-term symptoms and long-term complications of hypoparathyroidism. Our goal is to provide a PTH replacement therapy that normalizes serum and urinary calcium, as well as serum phosphate levels, and improves quality of life.